
SARS-CoV-2 RT-PCR

INTENDED USE
The SARS-CoV-2 RT-PCR kit is an in vitro diagnostics kit for the qualitative detection of the RNA of the
Severe Acute Respiratory Syndrome-related Coronavirus 2, taking as starting sample the RNA from human
clinical samples of different origin, such as, naso- and oropharyngeal swabs, bronchoalveolar lavages (BAL)
and saliva. It is based on a multiplex one-step real-time RT-PCR assay, using primers and fluorescencelabeled
probes specific for the gene targets N (independent regions N1 and N2) and E from SARS-CoV-2,
following the methodology recommended by the WHO.
The kit is designed for the universal detection of SARS-like coronaviruses with the primers and probe sets of
the E gene (Corman et al, 2020) and for the specific detection of SARS-CoV-2 with the primers and probe
sets of the N gene (N2 probe from the CDC protocol).
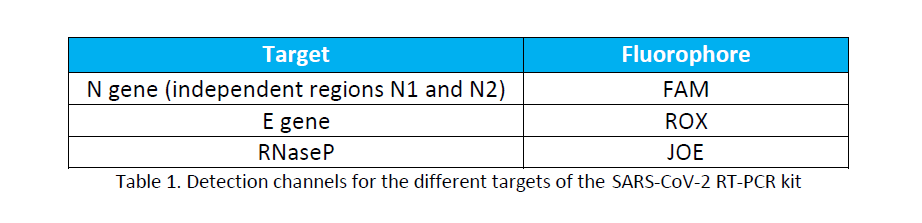
Specific fluorescent primers and probes are also included for the simultaneous detection of the human
RNaseP gene as internal quality control of the starting and amplification material. The detection channels
of the different targets are:
Microbiological status: Non-sterile product.